
A Guide to Hydrophobic and Oleophobic Coatings
Ever notice that when a few droplets of water land on your smartphone’s screen, they bead up as if floating on the surface? Or that fingerprints wipe away with a dry cloth, no cleaner needed? This is because the glass on most smartphones is treated with special coatings that repel water and oil. These hydrophobic (water repelling) and oleophobic or lipophobic (oil repelling) coatings are often called “self-cleaning” coatings because of how they shed water droplets and resist greasy fingerprints. Hydrophobic and oleophobic coatings are widely used in optical applications because they help protect optical surfaces and reduce the need for frequent cleaning.

Most people are familiar with these coatings because of their use on smartphones. Since fingers are used to activate devices with projected capacitive screens (multi-touch screens) or capacitive touch screens (single touch screens), oil and debris can build up on the surface. Frequent touching not only leaving the screen dirty but also reduces the device’s responsiveness and performance. Hydrophobic and oleophobic coatings prevent water and oil from wetting or sticking to the glass, making it easier to wipe away build-up. In addition to keeping the screen cleaner, eliminating the need to scrub, frequently wipe or use a solvent to remove dirt increases its longevity.
Smartphones are the most well-known application for hydrophobic and oleophobic coatings. However, since these coatings are usually optically transparent, they are used widely throughout consumer, industrial, and scientific products to improve optical surfaces’ ruggedness. Automotive, computing, life sciences, solar, and biotechnology are a few of the sectors utilizing these diverse coatings.
How Hydrophobic and Oleophobic Coatings Work
When a droplet of fluid rests on a flat surface in a gaseous environment, there is an adhesive force between a liquid droplet and a solid surface defined by the liquid-surface and liquid-gas interface. A droplet that clings tightly to the surface is said to wet it. The extent to which a droplet wets a surface is characterized by the contact angle between the surface and the droplet (see Figure 1).
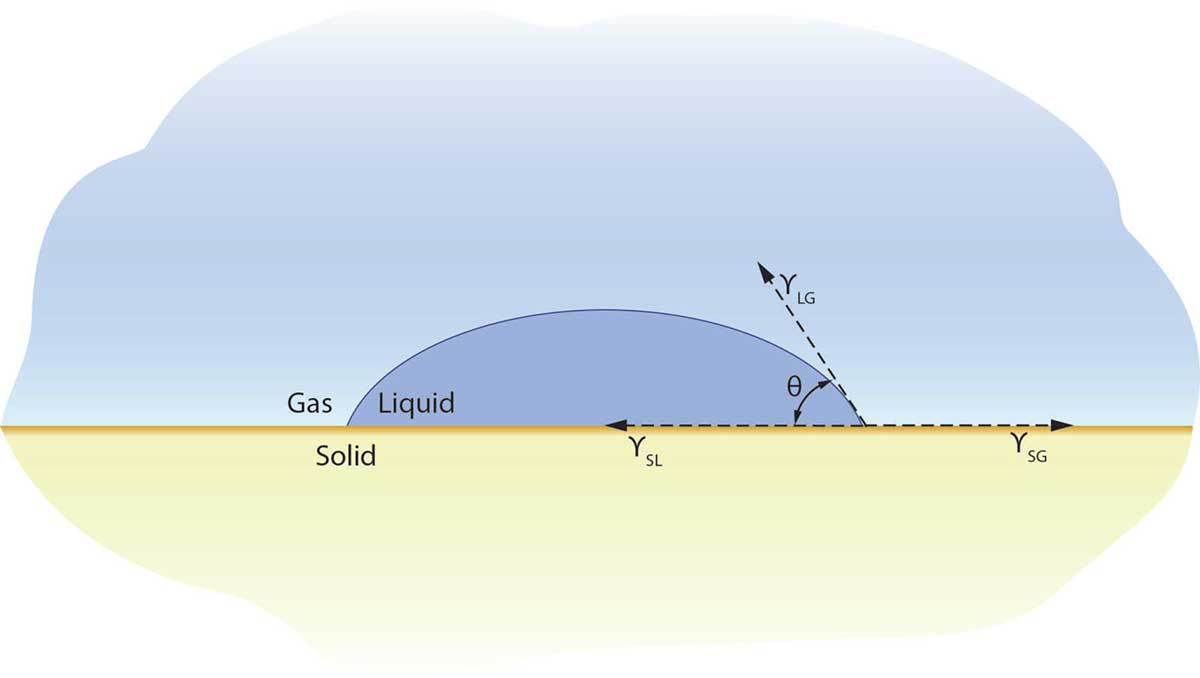
Thomas Young defined the contact angle according to the forces (interfacial tensions) acting on the droplet as follows.
ϒSG = ϒSL + ϒLG * Cos (θ)
where
ϒSG = solid surface free energy (or solid-gas interfacial tension)
ϒSL = solid-liquid interfacial free energy (or solid-liquid interfacial tension)
ϒLG = liquid surface free energy (or liquid-gas interfacial tension)
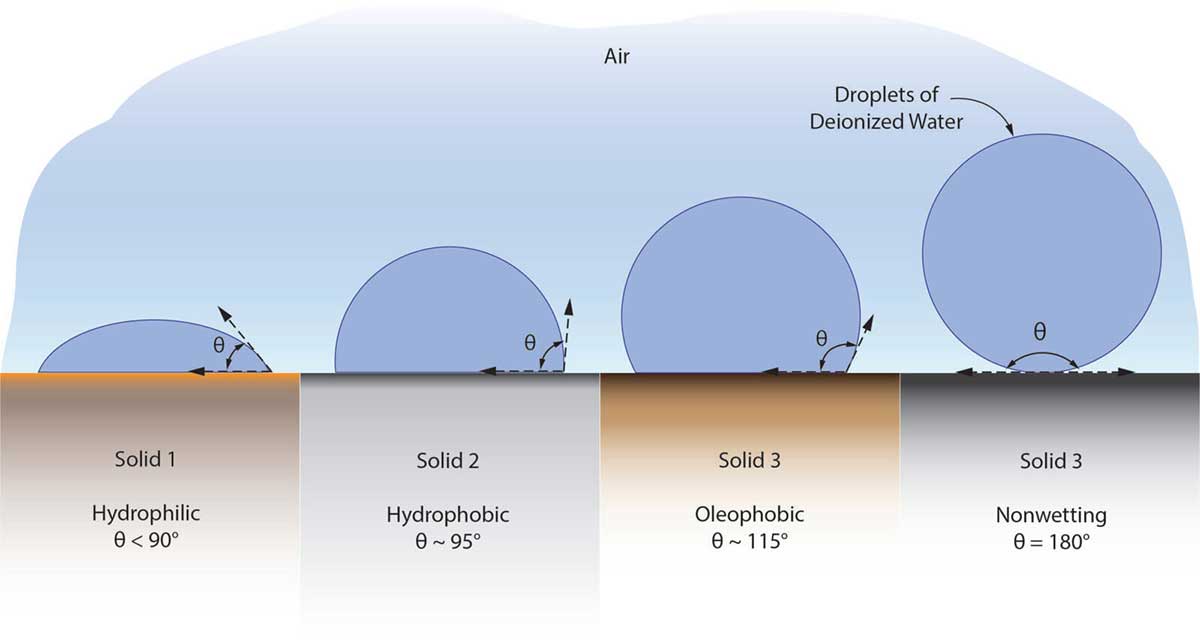
A special instrument called a contact angle goniometer or tensiometer is used to measure the contact angle between a droplet and a surface. If the contact angle is less than 90°, the liquid easily wets the surface. Contact angles between 90° and 180° indicate the liquid does not easily wet the surface and an angle of 180° is non-wetting.
In the case of a wetted surface, the liquid sticks, clinging to the surface, spreading. Water that wets glass will leave streaks, and as the water evaporates, the salts and dirt left behind resulting in a water ring.
The degree of wetting is determined by complex molecular interactions between the surface and liquid. A straightforward way of thinking about it for water is, since water is a polar molecule, wetting depends on whether the water molecules are attracted to surface molecules more than its own water molecules. If they are not, water molecules organize themselves into a droplet creating more liquid surface tension, a tighter droplet, and a wetting angle greater than 90°. This is a hydrophobic surface.
Hydrophobic versus Oleophobic Coatings
As their names imply, hydrophobic coatings shed water while oleophobic coatings shed oils. Unlike water, oil and organic fluids tend to be non-polar or only slightly polar. Thus, oils have a lower surface free energy—about a third of the surface free energy of water. So, oleophobic surfaces must also be very non-polar.
Oleophobic coatings can be characterized by the contact angle of a droplet of short-chain alkane on a surface. With n-hexadecane, a standard short-chain alkane testing fluid, contact angles between 60° and 80° indicate an oleophobic.
The contact angle for a droplet of deionized water can also be used to indicate oleophobicity. As a rule of thumb, if the contact angle with deionized water is greater than 90°, the surface is hydrophobic. Contact angles between 105° and 120° indicate an oleophobic surface. This means all oleophobic surfaces are also hydrophobic, while some hydrophobic surfaces are not oleophobic.
Advantages of Hydrophobic & Oleophobic Coatings
Hydrophobic and oleophobic coatings offer several advantages. As discussed, smartphone displays typically have an oleophobic coating for protection. These coatings often meet MIL-C-675-C durability requirements, which means they exhibit a degree of resistance to abrasion and adhesion. This added layer of security protects the substrate from scratches and prevents adhesives from sticking to them.
Fingerprints are ugly, but they can also degrade optical performance. Oils don’t adhere to an oleophobic surface as well as they do to an untreated surface. In addition to their slick, grease-resistant nature, oleophobic coatings are also considered “self-cleaning.” However, it is a misnomer to call hydrophobic and oleophobic coatings “water repellant” or “oil repellant.” They don’t’ actually repel water or oil; they simply don’t attract it. Water droplets roll off the treated surface and, in the process, collect dirt and debris removing them without the need for manual cleaning.
Perhaps one of the most significant advantages to hydrophobic and oleophobic coatings is that they reduce the need to clean the surface. Frequently wiping surfaces to remove spots and dirt increases the likelihood of scratches and other damages.
Applications
A common type of oleophobic coating is fluoropolymer deposited on a substrate. Their high lubrocity gives them a slippery feel. Polytetrafluoroethylene (PTFE) or Teflon — synthetic fluoropolymer of tetrafluoroethylene known for its use on nonstick cookware—is one such coating. Its contact angle with n-hexadecane is typically 65°.

Fluoropolymer-based solids and other non-polar polymers are commonly used as oleophobic coating displays. They are applied using vacuum vapor deposition making them thin with high light transmissibility. They are sufficiently optically transparent for use in a variety of applications. Like smartphone displays, eyeglass lenses often use oleophobic coatings to resist fingerprints and eliminate streaking. The hydrophobicity sheds water and helps with antifogging.
Nearly any optical display is a candidate for these coatings, but they are especially beneficial for touch displays such as ATMs, information kiosks, interactive displays, ticketing and check-in systems, touch-based control systems and personal electronic devices including laptops.
Although the automotive industry has resisted using oleophobic coatings due to cost sensitivity, incorporating in-cabin displays and external cameras to car designs means the coatings are finding more applications in the industry. Backup cameras are one such place where a “self-cleaning” coating has its advantages.
Similarly, hydrophobic and oleophobic coatings are beneficial for endoscopic cameras used for medical procedures and oral cameras used by dentists.
Another prominent use of hydrophobic and oleophobic coatings has been in the solar industry. Solar panels are exposed to the weather. Water droplets, dirt, and debris can impede their performance. Coating these large glass surfaces helps keep them clean and maintains their optical performance between cleanings. It also protects the surfaces from scratches during cleaning procedures.
High-end optics have also incorporated hydrophobic and oleophobic coatings. For example, broadband anti-reflective coatings (BBAR) may use hydrophobic and oleophobic coatings. In conjunction with shedding liquids, the coatings offer an extra layer of protection for the thin dielectric anti-reflective layers. While hydrophobic and oleophobic coatings may not be ideal for highly sensitive optical devices, they can be used on a wide range optical components such as mirrors, lenses and filters.
Overall, hydrophobic and oleophobic coatings offer many advantages with few drawbacks for a variety of applications. They should be considered for any application where liquid shedding and a degree of self-cleaning is desirable.





